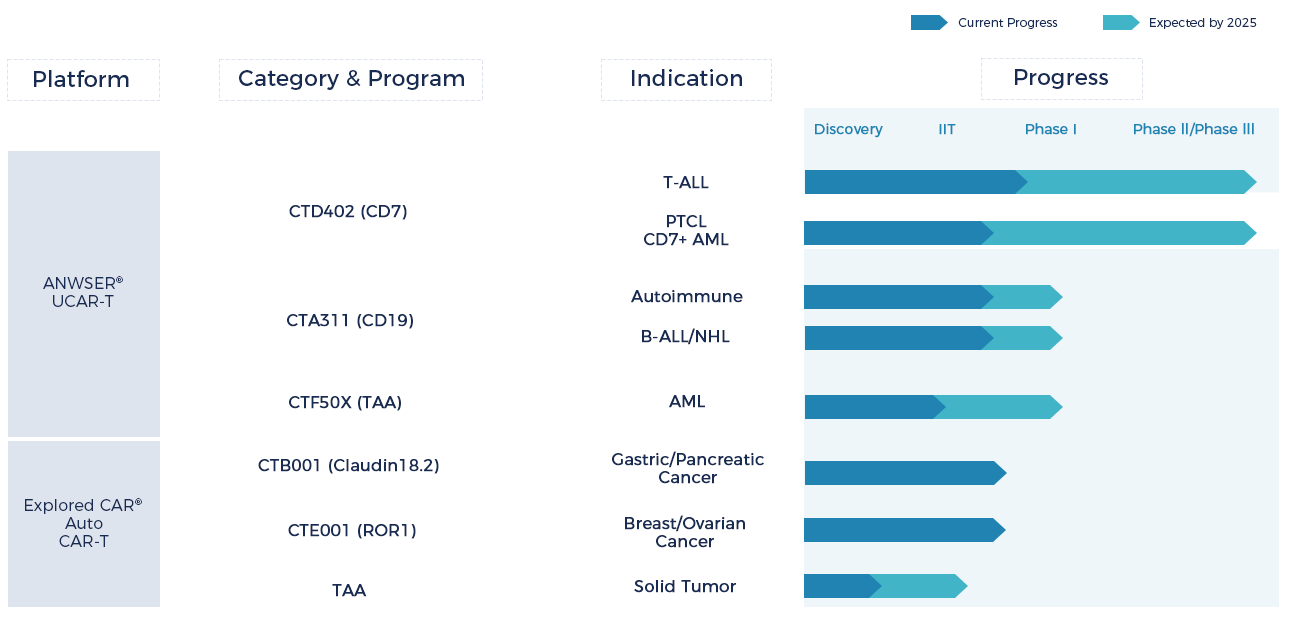
Pipeline
Our pipelines focus on oncology and autoimmune diseases
Technology Platforms
Our platforms 鈥 featuring ANSWER庐 CAR, Explored CAR庐 and RVVpacReady庐
ANSWER庐 2nd Gen UCAR-T
Onco & Non-onco
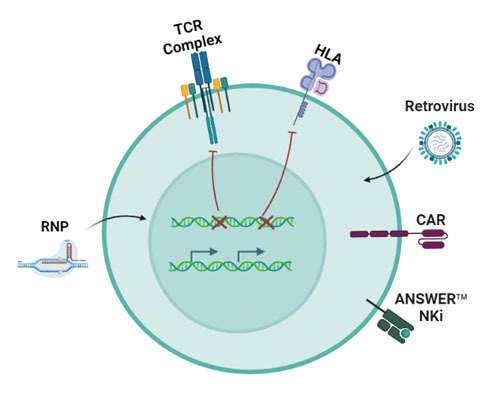
NK inhibition strategy: KO TCR, HLA and overexpress NK inhibitory molecule
Explored CAR庐
Solid Tumor
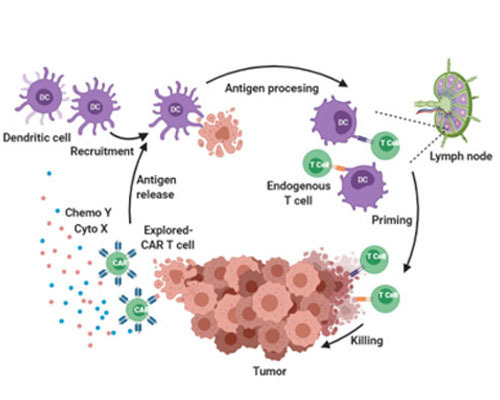
Solid tumor Armored CAR-T strategy: Secrete payload cytokine and chemokine to evoke in situ immune response
RVVpacReady庐
Viral Vector
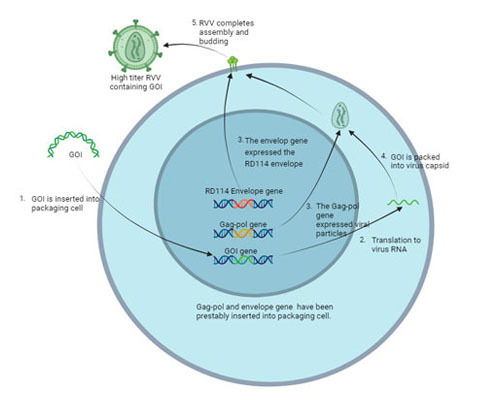
Higher harvest titer and transduction efficiency with lower COGS and impurities
Publications
Recently published articles
Chimeric antigen receptor (CAR)-T cell therapies for T-cell malignancies face significant challenges, including cannibalism among CAR-T cells and product contamination from cell explosion. Allogeneic CAR-T cells generated from healthy donor T cells can provide ready-to-use, blast-free therapeutic products, but their application may be complicated by graft-versus-host disease (GvHD) and host rejection. Here, we developed CD7-targeting CAR-T cells (RD13-01) from healthy donors that were genetically modified to resist cannibalism, GvHD, and allorejection and to enhance antitumor function . A phase I clinical trial (NCT04538599) enrolled 12 patients (11 with T-cell leukemia/lymphoma and 1 with CD7-expressing acute myeloid leukemia). All patients achieved the prespecified endpoint, and 11 patients were evaluated for efficacy. No dose-limiting toxicities, GvHD, immune effector cell-related neurotoxicity, or severe cytokine release syndrome (鈮rade 3) were observed. After 28 days of infusion, 81.8% (9/11) of patients experienced objective response, with a complete response rate of 63.6% (7/11, including AML patients). Three responding patients received allogeneic hematopoietic stem cell transplantation. At a median follow-up of 10.5 months, 4 patients remained in complete response. Reactivation of cytomegalovirus (CMV) and/or Epstein-Barr virus (EBV) was observed in several patients, one of whom died from EBV-related diffuse large B-cell lymphoma (DLBCL). Expansion of CD7-negative normal T cells was detected after infusion. In summary, we present the first report of a phase I clinical trial using healthy donor-derived CD7-targeting allogeneic CAR-T cells to treat CD7+ hematological malignancies. Our results demonstrate the encouraging safety and efficacy of RD13-01 allogeneic CAR-T cells for the treatment of CD7+ tumors.
Purpose: Autologous chimeric antigen receptor T (CAR-T) cell therapy is an effective treatment for relapsed/refractory acute lymphocytic leukemia (r/r ALL). However, the use of autologous CAR-T cells with certain characteristics may delay the availability of therapy. Another problem caused by antigen escape after relapsed single-target CAR-T treatment. Therefore, we aim to develop CRISPR-edited universal off-the-shelf CD19/CD22 dual-targeting CAR-T cells as a novel therapy for the treatment of refractory ALL. PATIENTS AND METHODS: In this open-label, dose-escalation phase I study, a universal CD19/CD22-targeted CAR-T cell (CTA101) CRISPR/Cas9 disrupted the TRAC region and the CD52 gene to avoid host immune-mediated rejection was injected. /r All in the patient's body. Safety, efficacy, and CTA101 cell kinetics were assessed. Results: CRISPR/Cas9 technology mediates efficient, high-fidelity gene editing and production of universal CAR-T
Background: T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) are highly aggressive T-lineage malignancies characterized by an infiltration of immature T cells in the bone marrow (BM) and peripheral blood (PB) or extramedullary organ involvements. Effective treatments were elusive, and the long-term survival of patients with refractory/relapsed (R/R) T-ALL/LBL remains poor despite allogeneic hematopoietic stem cell transplantation (allo-HSCT). CAR-T therapies for T-cell malignancies remain underdeveloped due to the shared antigenicity between normal and malignant T cells. CD7 is highly expressed on the surface of T-ALL/LBL T cells and is considered a viable CAR-T therapeutic target. However, CD7-targeting immunotherapies may be compromised by T-cell fratricide and extermination. And one of the biggest challenges with autologous CAR-T cell therapy is to isolate a sufficient number of healthy T cells from malignant T cells. Thus, clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR9-associated protein 9 (Cas9)-mediated gene ablation has been used to produce universal CD7-targeted CAR T cells (UCD7-CAR) from suitable healthy donors that no longer express CD7 and TCR. In vitro killing tests, animal tests, and phase 1 clinical trials have preliminarily verified the efficacy and safety of universal CD7 CAR-T cells. Here we investigated the safety and efficacy of the universal CD7 CAR-T therapy in phase 2 clinical trial(NCT05454241) for pediatric R/R T-ALL/LBL patients.
Background: T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) are highly aggressive T-lineage malignancies characterized by an infiltration of immature T cells in the bone marrow (BM) and peripheral blood (PB) or extramedullary organ involvements. Effective treatments were elusive, and the long-term survival of patients with refractory/relapsed (R/R) T-ALL/LBL remains poor despite allogeneic hematopoietic stem cell transplantation (allo-HSCT). CAR-T therapies for T-cell malignancies remain underdeveloped due to the shared antigenicity between normal and malignant T cells. CD7 is highly expressed on the surface of T-ALL/LBL T cells and is considered a viable CAR-T therapeutic target. However, CD7-targeting immunotherapies may be compromised by T-cell fratricide and extermination. And one of the biggest challenges with autologous CAR-T cell therapy is to isolate a sufficient number of healthy T cells from malignant T cells. Thus, clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR9-associated protein 9 (Cas9)-mediated gene ablation has been used to produce universal CD7-targeted CAR T cells (UCD7-CAR) from suitable healthy donors that no longer express CD7 and TCR. In vitro killing tests, animal tests, and phase 1 clinical trials have preliminarily verified the efficacy and safety of universal CD7 CAR-T cells. Here we investigated the safety and efficacy of the universal CD7 CAR-T therapy in phase 2 clinical trial(NCT05454241) for pediatric R/R T-ALL/LBL patients.
Although autologous CAR-T therapy targeting CD19+ B-cell has proven efficacy in hematological malignancies. The challenges such as undesirable waiting time, possible manufacturing failures and high cost, remain to be solved. In addition, antigen-loss/downregulation leads to treatment failure after single-target CAR-T therapy. To tackle these issues, we developed a universal CD19/CD22-targeting CAR-T cell with the TRAC region and CD52 gene disrupted using CRISPR/Cas9 technology. Pre-clinical data demonstrated the safety of CRISPR gene editing and the anti-leukemia ability of CTA101.
Contact Us





